Acid-Alkaline Balance
What is the alkaline-acid balance in our body? In a nutshell, it is the balance between base (alkaline) substances and acidic substances in the body. There are many substances the body uses to build, repair, and function; these are amino acids, enzymes, sugars, fatty acids, vitamins, minerals, and trace elements. Each of them has a specific function, however, each substance is either an acid or a base (alkaline). Acids and alkaline have opposing yet complementary characteristics, and the body needs both to function properly. When acids and alkaline substances are present in balanced quantities, then the body is considered to be in Acid-Alkaline Balance, also referred to as Homeostasis.
Healthy and Unhealthy pH Levels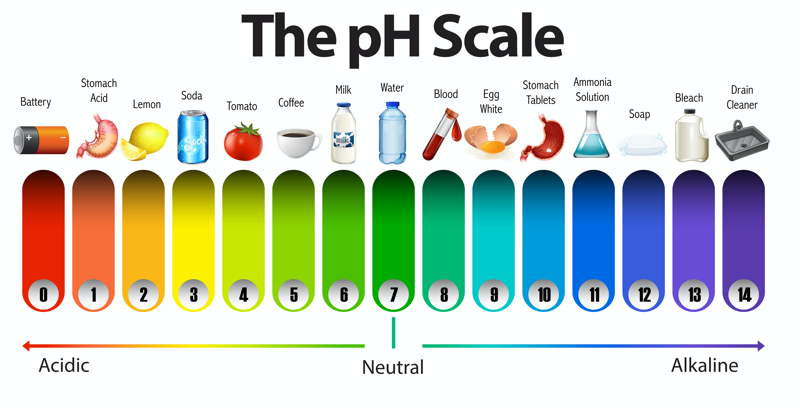
Our body is designed to function optimally when our internal bio-chemical environment measures 7.39 pH. The range for this optimum is very small from a more acidic environment of 7.36 pH to a more alkaline environment of 7.42 pH. If the body’s internal environment is anywhere between 7 and 7.36 pH, the body is considered to be in a state of acidosis. If the body’s internal environment is anywhere between 7.42 and 7.8 pH, the body is considered to be in a state of alkalosis. If the body gets into a state in which these limits are exceeded, it can no longer function and death will result. Our blood pH is kept in a range of 7.35 to 7.45. The body acid-base balancing procedures will do whatever they need to to keep the blood pH in this tight range.
About Acids substances
Acids almost always are combined with an alkaline element in nature. When the alkaline component in the acid is strong, then the acid is considered weak. The alkaline balances the acidity. When the alkaline component is weak, then the acid is considered strong. Weak acids (volatile acids), which are mostly found in plant foods, can be more easily eliminated by the body. They are eliminated by the lungs through carbon dioxide (CO2) exhalation and breath moisture. There is no limitation on how much volatile acid the lungs can eliminate. Strong acids on the other hand are fairly stable and don’t mix well with other elements and they are more difficult to neutralize and eliminate by the body. Strong acids are primarily processed by the kidneys with the skin as a secondary elimination system. The kidneys only have a certain amount of processing power and therefore have limitations. Acids that cannot be eliminated within a short period of time are stored in tissue in order to protect the body from exceeding the pH limits.
Uric, sulfuric, and phosphoric acids are the primary strong acids and come mostly from animal proteins. Citric, oxalic, pyruvic, and acetylsalicylic acids are the primary weak acids and come mostly from plants (carbohydrates and vegetable proteins).
Optimal pH Levels in The Body
All functions in the body start at the cellular level. Each function in the body operates optimally at a specific pH level (not necessarily all at the same pH level). When that pH level in the cell is off by even just a little, the chemical reactions are diminished, inhibited, or not possible depending on how much the pH deviates from the optimal level of 7.36 – 7.42. Chronic build-up of metabolic acids in the body leads to chronic low-grade acidosis, the most common acid-alkaline imbalance. Acidosis can be due to aging when the kidneys’ ability to process acids and toxins diminishes; however, with today’s lifestyles and diets, this can be seen even in the very young. Dietary habits of overconsumption of acidic foods like animal proteins, refined grains, sugars, processed foods, caffeinated beverages, and alcohol leads to a slightly more acidic than normal internal environment in the body and provides a hospitable environment for many diseases.
How Our Body Adapts to Keep pH at Proper Levels
The body has a built-in feed back loop where the environment is checked for a variety of conditions and when things are out of balance, the body will do what it needs to in order to bring the environment back into balance. In the case of acidosis for example, the body will take the alkaline substances, mainly calcium, potassium, and magnesium, from its buffer systems (areas that can function without the substances for a short time), mainly the bones, the muscles, and tissues of internal organs. If this “borrowing” continues without replenishing the buffers through nutritious foods, then these organs and tissues cannot function properly themselves and disease sets in. The longer the plundering lasts, the harder it is for the body to recover and the more likely tissues and organs will fail.
Some examples of what can happen when the body is in a constant low-grade acidosis are:
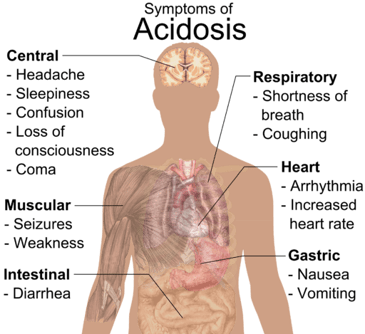 osteoporosis,
osteoporosis, - reduced bone formation,
- magnesium and potassium loss leading to hypertension and inflammation,
- pain,
- muscle wasting due to catabolism,
- reduced ability to repair cells, tissues and organs,
- irritation of the urinary tract and bladder,
- accelerated aging,
- increased oxidation and free radicals in the body,
- kidney stones,
- fluid retention,
- impaired tissue and organ function,
- digestive problems due to poor gut biome,
- increased viral, bacterial, and fungal infections
How can we prevent and / or reverse acidosis?
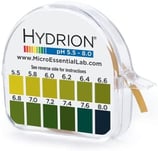
- Get tested for your pH levels. Using the proper incremental pH paper test strips is essential. Here is a link to one we recommend. Because the kidneys are responsible for eliminating the strong acids, the best indicator for the body’s acid levels is a urine test using the first morning’s urine.
- Foods that support acid-alkaline balance are vegetables, seeds, nuts, and fruits with green vegetables and root crops being particularly helpful.
If you know the pH value of your urine, you can determine how acidic your body is in general and eat according to your body’s needs. The more acidic your body is the more alkalizing food you should consume.
Want to learn more about your body’s acid-alkaline balance and how to address the acidity through dietary changes? Book an appointment with Dr. Evelin to discuss your personal pH levels. Dr. Evelin can help you get back on track through dietary changes.
To book an appointment with Dr. Evelin Valdez, ND, please visit https://booking.appointy.com/LotusWellness.
To set appointments with Dr. Evelin Valdez and view service pricing, please Use the QR code below by clicking the image or using your QR code reader
To connect with Dr. Evelin on Facebook, visit https://www.facebook.com/RidingHealer





